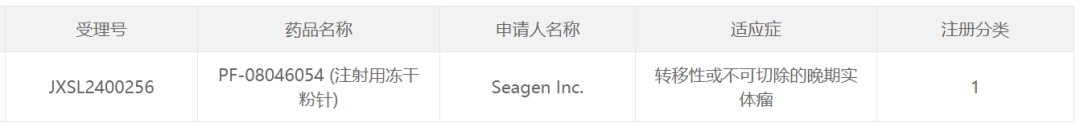
智通财经APP获悉,3月10日,中国国家药监局药品审评中心(CDE)官网最新公示,辉瑞(PFE.US)公司旗下Seagen申报的1类新药PF-08046054(注射用冻干粉针)获得临床试验默示许可,拟开发治疗转移性或不可切除的晚期实体瘤。公开资料显示,这是辉瑞在研的一款潜在“first-in-class”PD-L1靶向抗体偶联药物(ADC)。
截图来源:CDE官网
根据辉瑞公开资料介绍,PF-08046054(又称SGN-PDL1V、PDL1V)是一款靶向PD-L1的ADC,由抗PD-L1抗体、连接子和微管破坏剂MMAE(monomethyl auristatin E)组成,可通过直接细胞毒性、旁观者杀伤、免疫原性细胞死亡来发挥抗肿瘤活性。在临床前研究中,这款ADC产品在PD-L1低表达或表达异质性高的动物模型中也展现出抗癌活性。
在2024年欧洲肿瘤内科学会(ESMO)大会上,研究人员公布了PF-08046054的1期临床中期结果(SGNPDL1V-001研究)。
截至2024年3月6日,55名患者接受了治疗,中位年龄为60岁(范围24-72岁)。未见剂量限制性毒性(DLT);1.75 mg/kg为评价的最高剂量。最常见的治疗相关不良事件(TRAEs)为周围感觉神经病变、乏力、疲劳和恶心,大多数患者的严重程度为1-2级。有效性方面,研究者评估的所有剂量和肿瘤类型的客观缓解率(ORR)为27.3%(确定的ORR为12.7%),确定应答的中位持续时间为7.9个月。从1.25 mg/kg剂量开始观察到客观反应,且与PD-L1表达无关。